Weekly U.S. Influenza Surveillance Report
minusRelated Pages

Key indicators that track flu activity remain high but decreased for the second week in a row. Severity indicators (hospitalizations and deaths) remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults.
Viruses
Clinical Labs
The percentage of respiratory specimens testing positive for influenza at clinical laboratories decreased from 29.7% last week to 26.4% this week.
Public Health Labs
Numbers of influenza B/Victoria and A(H1N1)pdm09 viruses are approximately equal for the season overall, with continued increases in influenza A(H1N1)pdm09 viruses in recent weeks.
Virus Characterization
Genetic and antigenic characterization and antiviral susceptibility of influenza viruses collected in the U.S. are summarized in this report.
Illness
Outpatient Illness: ILINet
Visits to health care providers for influenza-like illness (ILI) decreased from 6.1% last week to 5.5% this week. All regions remain above their baselines.
Outpatient Illness: ILINet Activity Map

The number of jurisdictions experiencing high ILI activity decreased slightly from 46 last week to 45 this week.
Geographic Spread

The number of jurisdictions reporting regional or widespread influenza activity decreased from 51 last week to 50 this week.
Severe Disease
Hospitalizations
The overall cumulative hospitalization rate for the season increased to 52.7 per 100,000.
P&I Mortality
The percentage of deaths attributed to pneumonia and influenza is 6.9%, below the epidemic threshold of 7.3%.
Pediatric Deaths
20 influenza-associated pediatric deaths occurring during the 2019-2020 season were reported this week. The total for the season is 125.
All data are preliminary and may change as more reports are received.
A description of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component is available on the surveillance methods page.
Additional information on the current and previous influenza seasons for each surveillance component are available on FluView Interactive.
Key Points:
Outpatient ILI and clinical laboratory data remain elevated but decreased for the second week in a row. The percentage of specimens testing positive for both influenza A and influenza B viruses decreased.Overall, hospitalization rates remain similar to this time during recent seasons, but rates among school aged children and young adults are higher at this time than in recent seasons and rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.Pneumonia and influenza mortality has been low, but 125 influenza-associated deaths in children have been reported so far this season. This number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic.CDC estimates that so far this season there have been at least 32 million flu illnesses, 310,000 hospitalizations and 18,000 deaths from flu.Interim estimates of 2019-2020 flu vaccine effectiveness were released last week. So far this season, flu vaccines are reducing doctor’s visits for flu illness by 45% overall and 55% in children.Antiviral medications are an important adjunct to flu vaccine in the control of influenza. Almost all (>99%) of the influenza viruses tested this season are susceptible to the four FDA-approved influenza antiviral medications recommended for use in the U.S. this season.
U.S. Virologic Surveillance
Clinical Laboratories
The results of tests performed by clinical laboratories nationwide are summarized below. Data from clinical laboratories (the percentage of specimens tested that are positive for influenza) are used to monitor whether influenza activity is increasing or decreasing.
Week 8Data Cumulative since
September 29, 2019
(week 40)No. of specimens tested42,587948,064No. of positive specimens (%)11,249 (26.4%)
190,362 (20.1%)Positive specimens by type Influenza A 7,633 (67.9%)90,382 (47.5%) Influenza B 3,616 (32.1%)99,980 (52.5%)
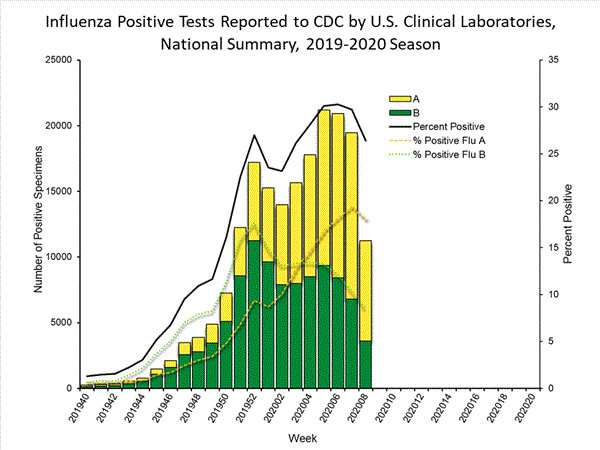
View Chart Data | View Full Screen
Public Health Laboratories
The results of tests performed by public health laboratories nationwide are summarized below. Data from public health laboratories are used to monitor the proportion of circulating viruses that belong to each influenza subtype/lineage.
Week 8Data Cumulative since
September 29, 2019
(week 40)No. of specimens tested 1,78362,234No. of positive specimens1,153 34,807Positive specimens by type/subtype Influenza A835 (72.4%)18,421 (52.9%) (H1N1)pdm09697 (94.8%)16,409 (91.6%) H3N238 (5.2%)1,511 (8.4%) Subtyping not performed100 501 Influenza B318 (27.6%)16,386 (47.1%) Yamagata lineage2 (0.7%)205 (1.6%) Victoria lineage277 (99.3%)12,657 (98.4%) Lineage not performed393,524
While influenza B/Victoria viruses predominated earlier in the season, during recent weeks, influenza A(H1N1)pdm09 viruses have been reported more frequently than B/Victoria viruses. For the season, the number of B/Victoria and A(H1N1)pdm09 viruses are approximately equal. The predominant virus varies by region. Regional and state level data about circulating influenza viruses can be found on FluView Interactive.
The predominant virus also varies by age group. Nationally, for the season overall, influenza B viruses are the most commonly reported influenza viruses among children and young adults less than 25 years, while A viruses are the most commonly reported influenza viruses among persons 25 years and older. In the most recent weeks, influenza A viruses are the most commonly reported influenza viruses in all but the school aged children and young adults (5-24 years old).
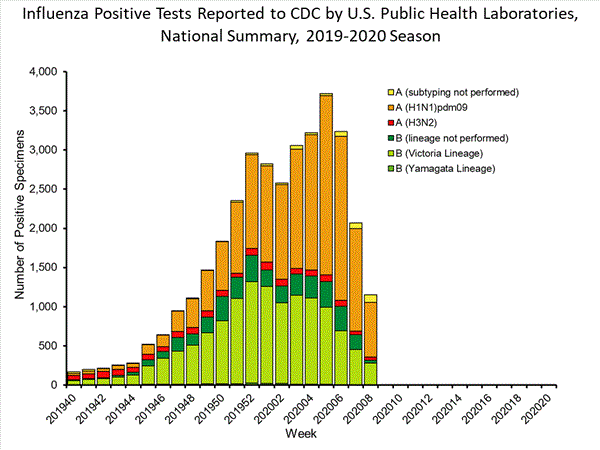
View Chart Data | View Full Screen
Additional virologic surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive: National, Regional, and State Data or Age Data
<!--
Novel Influenza A Virus:
One additional human infection with a novel influenza A virus was reported by Ohio. This person was infected with an influenza A(H1N2) variant (A(H1N2)v) virus and reported exposure to swine at an agricultural fair during the week preceding illness onset. The patient is a child < 18 years of age, was not hospitalized, and continues to recover from illness.
A total of 14 variant viruses have been reported to CDC during 2018. One of these has been an influenza A(H3N2) variant (A(H3N2)v) virus (Indiana [1]), and 13 have been A(H1N2)v viruses (California [6], Michigan [3], and Ohio [4]).
Early identification and investigation of human infections with novel influenza A viruses are critical so that the risk of infection can be more fully understood and appropriate public health measures can be taken. Additional information on influenza in swine, variant influenza infection in humans, and strategies to interact safely with swine can be found at http://www.cdc.gov/flu/swineflu/index.htm.
Additional information regarding human infections with novel influenza A viruses can be found at http://gis.cdc.gov/grasp/fluview/Novel_Influenza.html.
Influenza Virus Characterization
CDC performs genetic and antigenic characterization of U.S. viruses submitted from state and local health laboratories using Right Size Roadmap submission guidance. These data are used to compare how similar the currently circulating influenza viruses are to the reference viruses used for developing new influenza vaccines and to monitor evolutionary changes that continually occur in circulating influenza viruses. Antigenic characterization data are based on an animal model (influenza-naive ferrets), and do not reflect pre-existing protection provided by past influenza infections and vaccinations. Additional antigenic characterization studies involving people vaccinated with current influenza vaccines are conducted later in the season; these data account for pre-existing protection in different populations against circulating influenza viruses. Genetic and antigenic characterization data are not used to make calculations about vaccine effectiveness (VE). CDC conducts VE studies each year to measure the benefits of flu vaccines in people. Interim estimates of 2019-2020 flu vaccine effectiveness have been released.
CDC genetically characterized 1,787 influenza viruses collected in the U.S. from September 29, 2019, to February 22, 2020.
Virus Subtype or LineageGenetic CharacterizationTotal No. of Subtype/Lineage Tested CladeNumber (% of subtype/lineage tested) SubcladeNumber (% of subtype/lineage tested)A/H16066B.1A606 (100%)A/H3 4063C.2a386 (95.1%)2a1386 (95.1%) 2a20 2a302a403C.3a20 (4.9%)3a20 (4.9%)B/Victoria699V1A699 (100%)V1A0V1A.151 (7.3%)V1A.3648 (92.7%)B/Yamagata76Y376 (100%)
CDC antigenically characterizes a subset of influenza viruses by hemagglutination inhibition (HI) or neutralization based Focus Reduction assays (FRA). Antigenic drift is evaluated by comparing antigenic properties of cell-propagated reference viruses representing currently recommended vaccine components with those of cell-propagated circulating viruses. CDC antigenically characterized 287 influenza viruses collected in the United States from September 29, 2019, to February 22, 2020. These data are not used to make calculations about vaccine effectiveness (VE). CDC conducts VE studies each year to measure the benefits of flu vaccines in people.
Influenza A Viruses
A (H1N1)pdm09: 74 A(H1N1)pdm09 viruses were antigenically characterized by HI with ferret antisera, and all 74 (100%) were antigenically similar (reacting at titers that were within 4-fold of the homologous virus titer) to cell-propagated A/Brisbane/02/2018-like reference viruses representing the A(H1N1)pdm09 component for the 2019-20 Northern Hemisphere influenza vaccines.A (H3N2): 72 A(H3N2) viruses were antigenically characterized by FRA with ferret antisera, and 31 (43.1%) were antigenically similar to cell-propagated A/Kansas/14/2017-like reference viruses representing the A(H3N2) component for the 2019-20 Northern Hemisphere influenza vaccines.
Influenza B Viruses
B/Victoria: 131 B/Victoria lineage viruses, including viruses from both co-circulating sub-clades, were antigenically characterized by HI with ferret antisera, and 83 (63.4%) were antigenically similar to cell-propagated B/Colorado/06/2017-like reference viruses representing the B/Victoria component for the 2019-20 Northern Hemisphere influenza vaccines.B/Yamagata: 10 B/Yamagata lineage viruses were antigenically characterized by HI with ferret antisera, and all 10 (100%) were antigenically similar to cell-propagated B/Phuket/3073/2013-like reference viruses representing the B/Yamagata component for the 2019-20 Northern Hemisphere influenza vaccines.
CDC assesses susceptibility of influenza viruses to the antiviral medications including the neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) and the PA endonuclease inhibitor baloxavir using next generation sequence analysis supplemented by laboratory assays. Viruses collected in the United States since September 29, 2019, were tested for antiviral susceptibility as follows:
Antiviral MedicationTotal VirusesA/H1A/H3B/VictoriaB/YamagataNeuraminidase InhibitorsOseltamivirViruses Tested1,77660639969576 Reduced Inhibition1 (0.1%)(0.0%)(0.0%)1 (0.1%)(0.0%)Highly Reduced Inhibition3 (0.2%)3 (0.5%)(0.0%)(0.0%)(0.0%)PeramivirViruses Tested1,77660639969576 Reduced Inhibition(0.0%)(0.0%)(0.0%)(0.0%)(0.0%)Highly Reduced Inhibition4 (0.2%)3 (0.5%)(0.0%)1 (0.1%)(0.0%)ZanamivirViruses Tested 1,77660639969576 Reduced Inhibition2 (0.1%)(0.0%)(0.0%)2 (0.3%)(0.0%)Highly Reduced Inhibition(0.0%)(0.0%)(0.0%)(0.0%)(0.0%)PA Endonuclease InhibitorBaloxavir Viruses Tested1,92662648473779Reduced Susceptibility(0.0%)(0.0%)(0.0%)(0.0%)(0.0%)
A total of 416 additional viruses (199 A(H1N1)pdm09, 30 A(H3N2), and 187 B) collected in Alabama, Alaska, Florida, Illinois, Iowa, Louisiana, Massachusetts, Michigan, Nevada, New York, North Carolina, Pennsylvania, South Dakota, Virginia and Wisconsin were analyzed for resistance to neuraminidase inhibitors by pyrosequencing assay. Two (1.0%) of the 199 A(H1N1)pdm09 viruses tested had the H275Y amino acid substitution in the neuraminidase that confers resistance to oseltamivir and potential resistance to peramivir. No markers of resistance to neuraminidase inhibitors were detected in A(H3N2) and type B viruses tested.
<!--

View Chart Data | View Full Screen | View PowerPoint Presentation
Outpatient Illness Surveillance
ILINet
Nationwide during week 8, 5.5% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is above the national baseline of 2.4%.
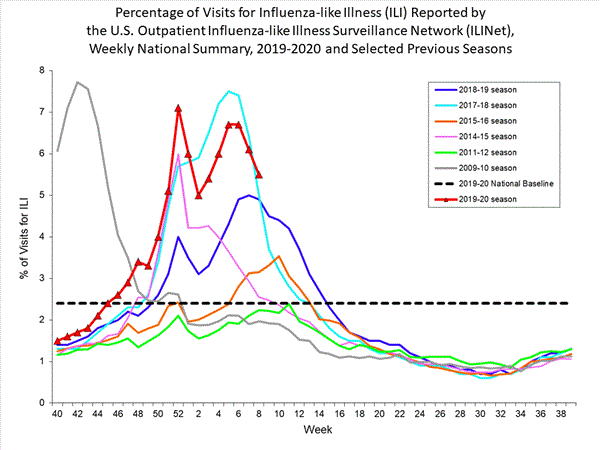
View Chart Data (current season only) | View Full Screen
On a regional level, the percentage of outpatient visits for ILI ranged from 3.6% to 8.8% during week 8. All regions reported a percentage of outpatient visits for ILI above their region-specific baselines.
ILI Activity Map
Data collected in ILINet are used to produce a measure of ILI activity* by state.
During week 8, the following ILI activity levels were experienced:
High – New York City, Puerto Rico, and 43 states (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Georgia, Hawaii, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming)Moderate – five states (Delaware, Nevada, North Dakota, Ohio, and Oregon)Low - the District of Columbia and one state (Idaho)Minimal - the U.S. Virgin Islands and one state (Florida)
[iframe]"[/iframe]
*Data collected in ILINet may disproportionally represent certain populations within a state, and therefore, may not accurately depict the full picture of influenza activity for the whole state. Differences in the data presented here by CDC and independently by some state health departments likely represent differing levels of data completeness with data presented by the state likely being the more complete.
Additional information about medically attended visits for ILI for current and past seasons:
Surveillance Methods | FluView Interactive: National, Regional, and State Data or ILI Activity Map
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists
The influenza activity reported by state and territorial epidemiologists indicates geographic spread of influenza viruses but does not measure the severity of influenza activity.
During week 8, the following influenza activity was reported:
Widespread – Puerto Rico and 48 states (Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, Virginia, Washington, West Virginia, Wisconsin, and Wyoming)Regional – one state (Oregon)Local – the District of Columbia and one state (Hawaii)Sporadic – the U.S. Virgin IslandsGuam did not report.
[iframe]"[/iframe]
Additional geographic spread surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
Influenza-Associated Hospitalizations
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in select counties in the Emerging Infections Program (EIP) states and Influenza Hospitalization Surveillance Project (IHSP) states.
A total of 15,319 laboratory-confirmed influenza-associated hospitalizations were reported by FluSurv-NET sites between October 1, 2019 and February 22, 2020; 10,439 (68.1%) were associated with influenza A virus, 4,797 (31.3%) with influenza B virus, 47 (0.3%) with influenza A virus and influenza B virus co-infection, and 36 (0.2%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 2,834 (94.1%) were A(H1N1)pdm09 virus and 179 (5.9%) were A(H3N2).
The overall cumulative hospitalization rate was 52.7 per 100,000 population which is similar to what has been seen during recent previous influenza seasons at this time of year. Rates in school aged children and young adults are higher than at this time in recent seasons, and rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.
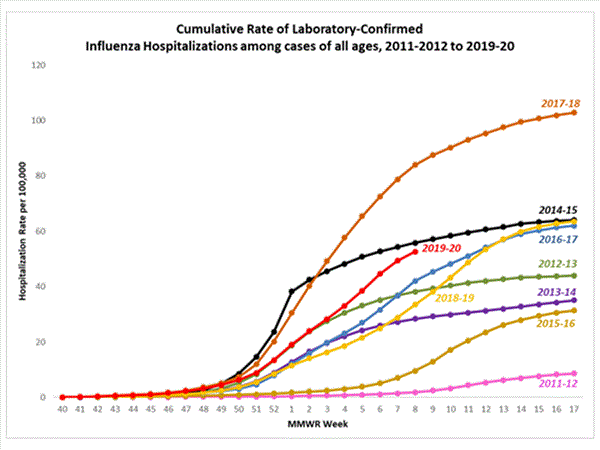
The highest rate of hospitalization is among adults aged ≥ 65, followed by children aged 0-4 years and adults aged 50-64 years.
Age Group2019-2020 Season
Cumulative Rate per 100,000 PopulationOverall52.70-4 years 80.15-17 years20.618-49 years28.650-64 years 69.165+ years132.0
Among 2,172 hospitalized adults with information on underlying medical conditions, 92.1% had at least one reported underlying medical condition, the most commonly reported were cardiovascular disease, metabolic disorder, obesity, and chronic lung disease. Among 397 hospitalized children with information on underlying medical conditions, 48.6% had at least one underlying medical condition; the most commonly reported was asthma. Among 381 hospitalized women of childbearing age (15-44 years) with information on pregnancy status, 24.1% were pregnant.
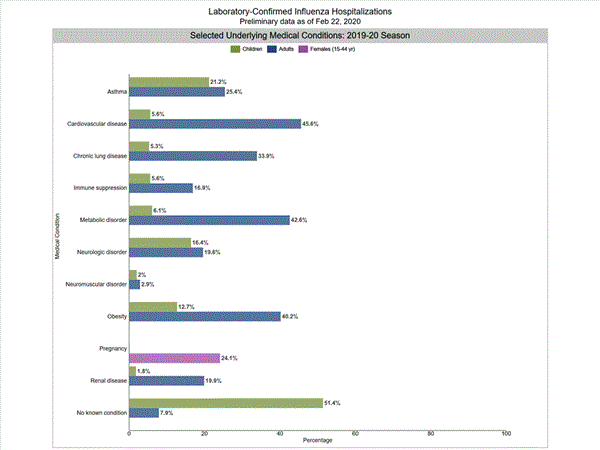
Additional hospitalization surveillance information for current and past seasons and additional age groups:
Surveillance Methods | FluView Interactive: Rates by Age or Patient Characteristics
Pneumonia and Influenza (P&I) Mortality Surveillance
Based on National Center for Health Statistics (NCHS) mortality surveillance data available on February 27, 2020, 6.9% of the deaths occurring during the week ending February 15, 2020 (week 7) were due to P&I. This percentage is below the epidemic threshold of 7.3% for week 7.
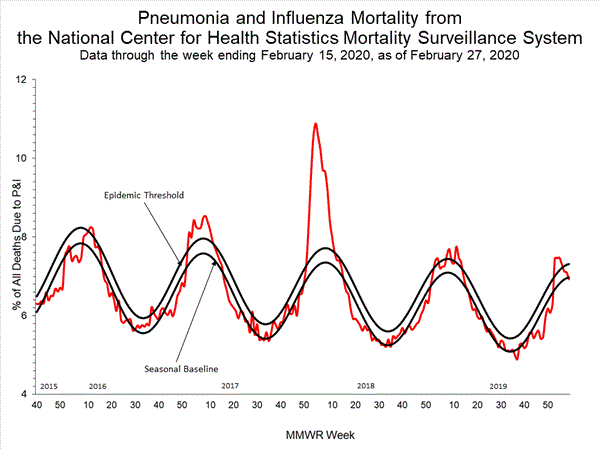
View Chart Data | View Full Screen
Additional pneumonia and influenza mortality surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
Influenza-Associated Pediatric Mortality
20 influenza-associated pediatric deaths occurring during the 2019-2020 season between weeks 52 and 8 (the weeks ending December 28, 2019 and February 22, 2020) were reported to CDC during week 8. 15 were associated with influenza B viruses, and four had a lineage determined; all B/Victoria viruses. Five were associated with influenza A viruses, and three were subtyped; all A(H1N1)pdm09 viruses.
Of the 125 influenza-associated pediatric deaths occurring during the 2019-2020 season and reported to CDC:
87 deaths were associated with influenza B viruses, and 18 had a lineage determined; all were B/Victoria viruses.38 deaths were associated with influenza A viruses, and 23 were subtyped; 22 were A(H1N1)pdm09 viruses and one was an A(H3) virus.
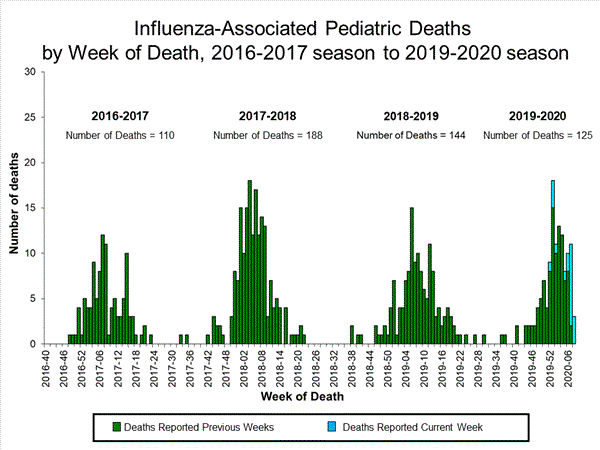
View Full Screen
Additional pediatric mortality surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics. To access these tools, visit http://www.cdc.gov/flu/weekly/fluviewinteractive.htm
National Institute for Occupational Safety and Health: Monthly surveillance data on the prevalence of health-related workplace absenteeism among full-time workers in the United States are available from NIOSH at https://www.cdc.gov/niosh/topics/absences/default.html
U.S. State and local influenza surveillance:Select a jurisdiction below to access the latest local influenza information
<!--smarker5-->
Alabama
Alaska
Arizona
Arkansas
California
Colorado
Connecticut
Delaware
District of Columbia
Florida
Georgia
Hawaii
Idaho
Illinois
Indiana
Iowa
Kansas
Kentucky
Louisiana
Maine
Maryland
Massachusetts
Michigan
Minnesota
Mississippi
Missouri
Montana
Nebraska
Nevada
New Hampshire
New Jersey
New Mexico
New York
North Carolina
North Dakota
Ohio
Oklahoma
Oregon
Pennsylvania
Rhode Island
South Carolina
South Dakota
Tennessee
Texas
Utah
Vermont
Virginia
Washington
West Virginia
Wisconsin
Wyoming
New York City
Puerto Rico
Virgin Islands
<!--emarker5-->
World Health Organization: Additional influenza surveillance information from participating WHO member nations is available through FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza located in Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia).
Europe: For the most recent influenza surveillance information from Europe, please see WHO/Europe and the European Centre for Disease Prevention and Control at http://www.flunewseurope.org/.
Public Health Agency of Canada: The most up-to-date influenza information from Canada is available at http://www.phac-aspc.gc.ca/fluwatch/
Public Health England: The most up-to-date influenza information from the United Kingdom is available at https://www.gov.uk/government/statistics/weekly-national-flu-reports
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
An overview of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component, is available at: http://www.cdc.gov/flu/weekly/overview.htm.